
LSU Chemistry Photo Contest
It's National Chemistry Week (Oct 20-26), and we're excited to host the LSU Chemistry Photo Contest! This year’s theme is “Picture Perfect Chemistry,” highlighting how chemistry contributes to photography and imaging.
LSU Chemistry faculty, staff, and students creatively captured chemistry in action—whether through vibrant chemical reactions, stunning imagery, or chemistry-inspired artwork—bringing picture perfect chemistry to life.
2024 "Picture Perfect Chemistry" Photo Contest Winner
Entry 16: "Illuminating the Invisible"
Submitted by: Simran Dhingra, Graduate Student, Vicente Research Group
The image offers a glimpse into the world of fluorescence. Simran synthesizes these compounds to visualize the hidden realm of cancer cells. Join her in exploring the beauty and brilliance of fluorescence as it reveals what lies beneath!

2024 Photo Entries
Entry 1: "Pink Pony Club"
Submitted by: Hannah Heinz, Graduate Student, Spivak Research Group
The image showcases Bis(thiobenzoyl) disulfide dissolved in THF. The compound’s absorption band is around 525 nm, which gives a vibrant pink color. This photo steals the spotlight and keeps the chemistry party going strong with its stunning hue!

Entry 2: "‘Rock’ you like a hurricane!"
Submitted by: Sajila Tanha, Graduate Student, Lee Research Group
Presenting our next rockstar photo—crystals of a novel ligand! Like nature’s own ‘rocks,’ these beauties show off their size and brilliance, a whirlwind outcome of Sajila’s synthesis! Chemistry never looked boulder!

Entry 3: "October Orange"
Submitted by: Jose Garfias, Graduate Student, Rivas Research Group
Spooky season is here, and so is Jose's “October Orange” reaction! The photo features a cyclization to form a lactam synthon. It is more than just an eerie sight; it’s a major building block in synthesizing a bioactive natural product!

Entry 4: "Transition States: The Chemistry Building by Day and Night"
Submitted by: Rosalia “Rosie Ace” Hernandez, Chemistry Senior
Rosie Ace captures LSU's chemistry buildings in two contrasting moods. By day, they blend harmoniously with the fall foliage, creating a peaceful and natural scene. At night, the dim lighting and solitary yellow bollards introduce a mysterious, reflective atmosphere, standing out as unusual elements in an otherwise quiet environment. Chemistry meets fall's beauty and night’s eerie charm!

Entry 5: "Tiny Machines in the World of Molecules"
Submitted by: Udyogi Conthagamage, Graduate Student, García-López Research Group
Chemistry graduate student Udyogi captured these artificial molecular shuttles, nanoscale machines engineered to transport molecules or ions along defined pathways. These tiny but mighty systems, powered by external stimuli like pH, ions, electric fields, or light, offer potential breakthroughs in drug delivery, molecular computing, and nanotechnology!

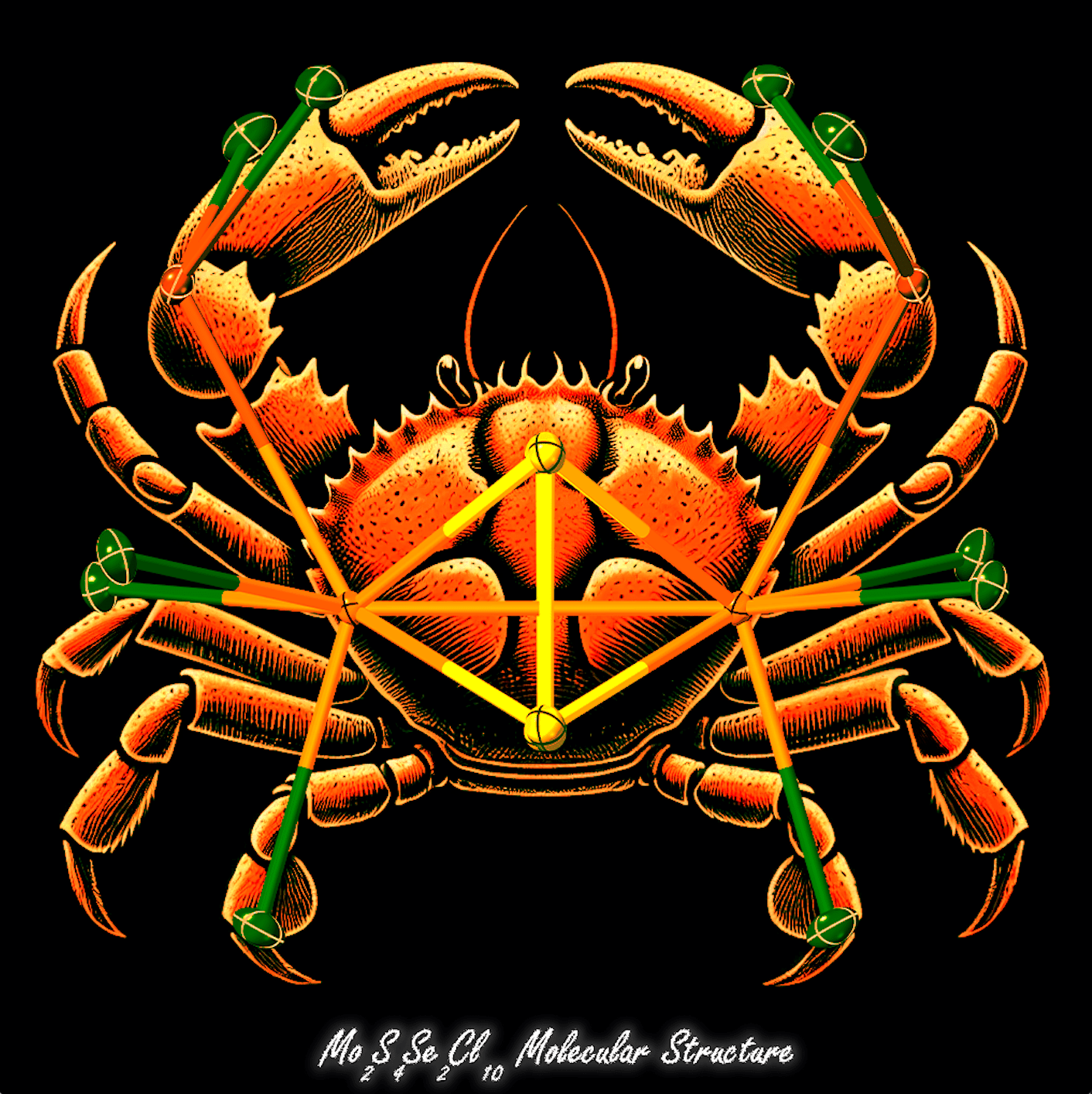
Entry 6: "The Structural Elegance of Mo₂S₄Se₂Cl₁₀"
Submitted by: Thimira Kandabadage, Graduate Student, Baranets Research Group
Get ready for some crab-tastic chemistry! Thimira's image presents a crab-like molecular geometry of Mo₂S₄Se₂Cl₁₀, where molybdenum, sulfur, selenium, and chlorine atoms are intricately arranged. This unique atomic configuration balances symmetry and complexity, illustrating the fusion of scientific precision with artistic flair.
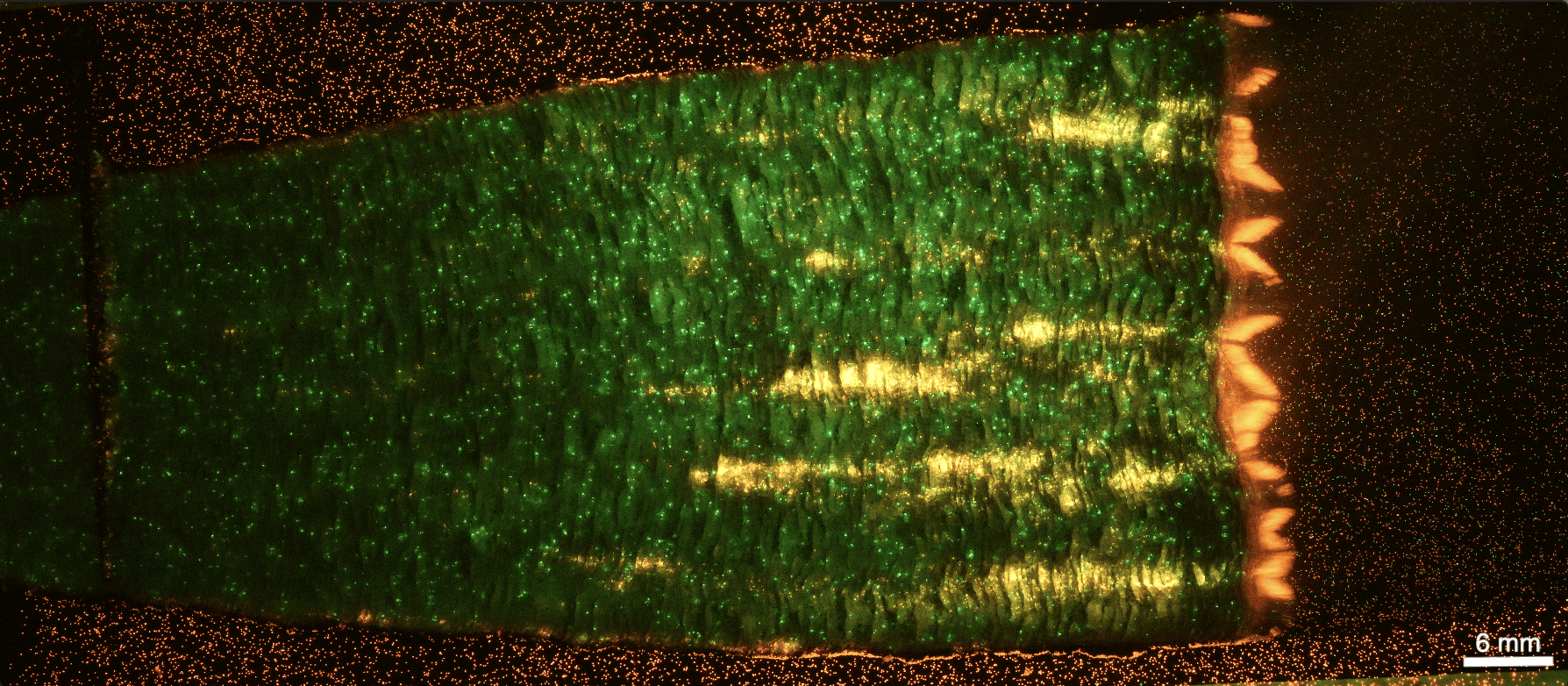
Entry 7: "Glow with the Flow"
Submitted by: Alexandra Aucoin, Graduate Student, Pojman Research Group
Check out this stunning submission by Alexandra for our NCW photo contest! The image captures the polymerization of thin-layer monomer solutions containing Copsheric fluorescent microspheres. As the polymerization front moves, the vibrant green and orange spheres light up, creating a mesmerizing glow.
Entry 8: "Tiger Spirit"
Submitted by: Alex Behm, Graduate Student, Rivera Research Group
We have spirit—yes, we do! Alex captured samples submitted to the Mass Spectrometry Facility, showcasing LSU’s signature purple and gold—a winning combination both on the field and in the lab!

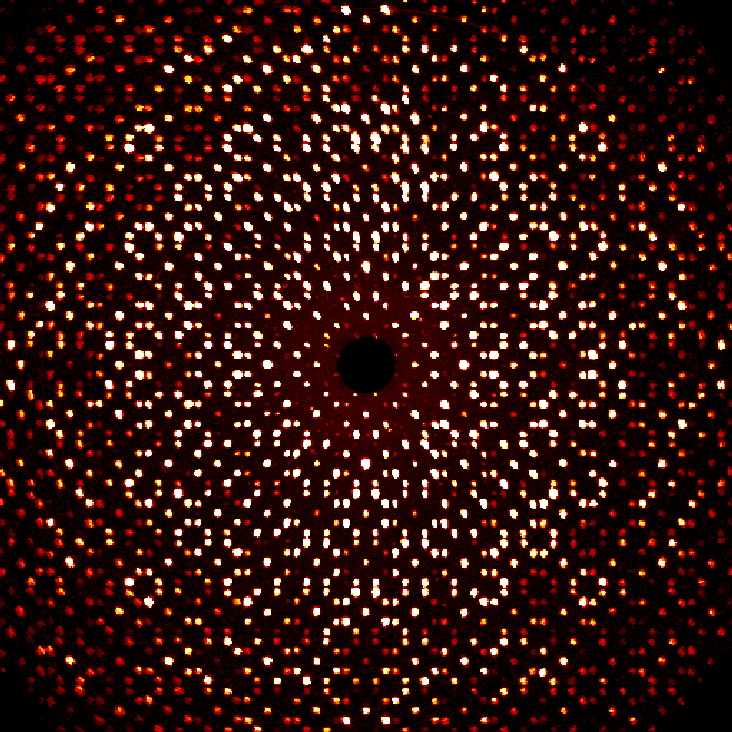
Entry 9: "Perfect Twin"
Submitted by: Spencer Watts, Graduate Student, Baranets Research Group
Prepare to be captivated by this single crystal X-ray diffraction precession image of a twinned barium oxyarsenide crystal! The perfect symmetry and intricate details will draw you in and leave you mesmerized—it's like seeing double with a touch of crystal magic!
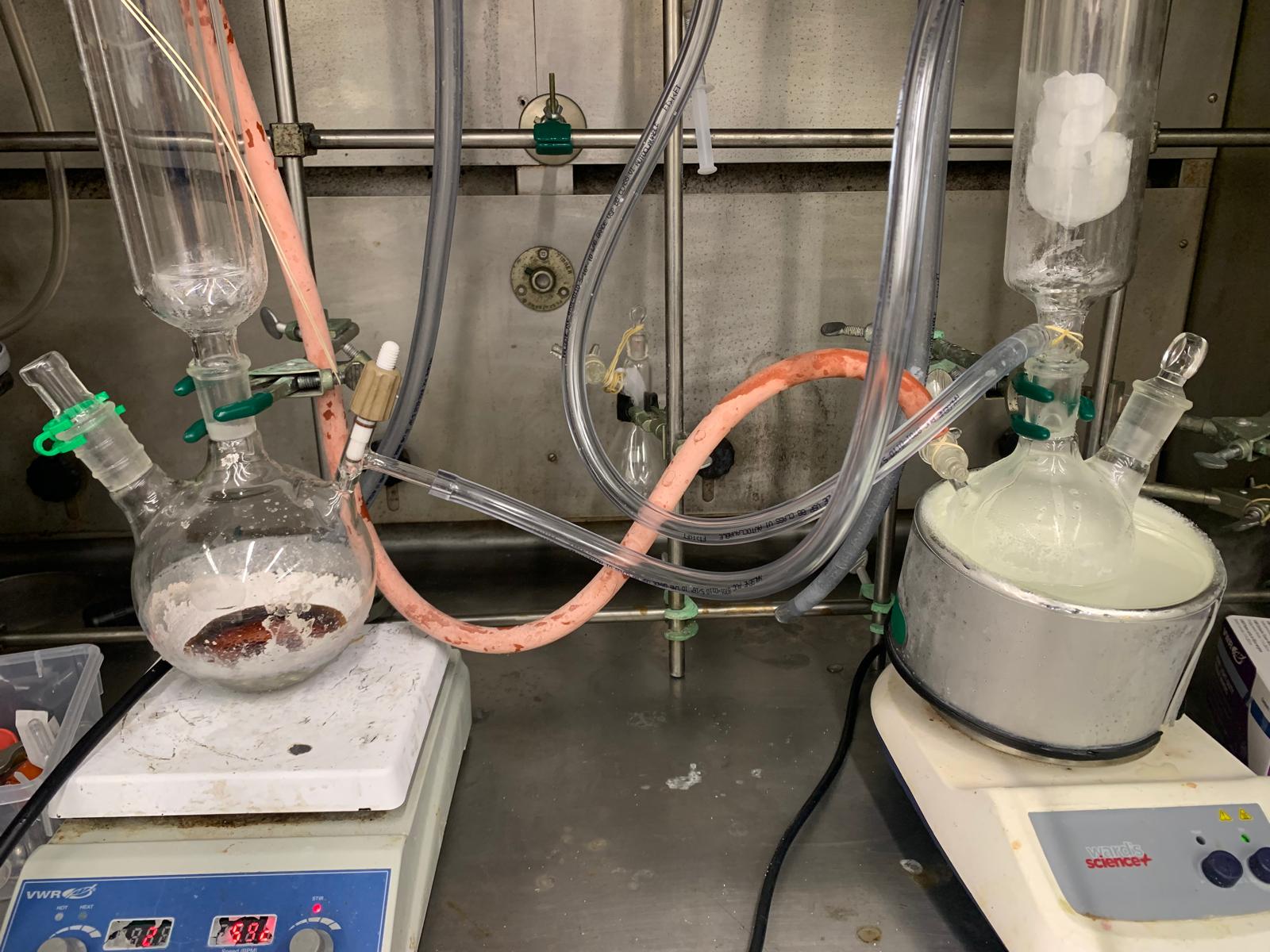
Entry 10: "Solvating Electrons, Drying Ammonia"
Submitted by: Prof. Clifton Wagner and Graduate Student Al Maruf
Did you know so-called "anhydrous" ammonia gas contains a significant amount of water contamination? Therefore, to use ammonia in a reaction that is incompatible with water, it must be dried first. The Wagner group shows how this can be done by condensing the ammonia onto a chunk of sodium, forming an intensely colored solution of solvated electrons, then evaporating the dry ammonia and recondensing it onto the desired reactant.
Entry 11: "Preparation of Mn(HMDS)2"
Submitted by: Fahmida Islam, Graduate Student, Wagner Research Group
Check out this snapshot showing the preparation of Mn(HMDS)₂ from LiHMDS and MnCl₂. After distillation, the air-sensitive pure Mn(HMDS)₂ is collected as colorless solids, while the impurities remain as brown material.

Entry 12: "Bright Science at the Vicente/Smith Research Group"
Submitted by: Sebastian Oloo, Graduate Student, Vicente & Smith Research Group
Diving into a spectrum of possibilities with fluorescent dyes. Every color tells a unique story, highlighting the magic of different molecular interactions.

Entry 13: "Rainbow"
Submitted by: Kateryna Tsymbal, Analytical Stockroom Coordinator
This vibrant photo comes straight from our analytical teaching labs! Featuring solutions of various salts, one pH buffer, an indicator, and the result of an overshot titration. No rain is required to find this rainbow!

Entry 14: "Thiodithiazyl Crystals"
Submitted by: Uthpala Nayomi Ekanayaka Arachchige, Graduate Student, Wagner Research Group
This image shows the reflux setup for the "Thiodithiazyl" reaction, where you can see crystals forming and collecting on the condenser walls. It showcases the vibrant chemistry at play in the creation of reddish-gold crystals.

Entry 15: "Crystal of Providence"
Submitted by: Olha Pokhvata, Graduate Student, Baranets Research Group
Feast your eyes on EuZn₂As₂! Crystallizing in a hexagonal system, this crystal forms a hexagonal structure with a triangular core reminiscent of the Eye of Providence, watching over with sharp precision.

Entry 16: "Illuminating the Invisible"
Submitted by: Simran Dhingra, Graduate Student, Vicente Research Group
The image offers a glimpse into the world of fluorescence. Simran synthesizes these compounds to visualize the hidden realm of cancer cells. Join her in exploring the beauty and brilliance of fluorescence as it reveals what lies beneath!
